Mimicking Blood for Carbon Dioxide Capture and Release | Basics
Just another wonderful case of biomimicry
Hello, 👋
In today's business landscape, sustainability drives innovations across various sectors. One exciting development is using haemoglobin-inspired technologies for carbon capture. By mimicking haemoglobin's natural ability to bind and transport gases, scientists are creating efficient methods to sequester carbon dioxide. In this article, we explore Capturing Carbon by Mimicking Haemoglobin: Trends, Challenges, and Opportunities. Happy reading!
If you are new to carbon economy, scan through the following article first.
Basics: Business of Carbon
Hello, 👋 The business landscape is in a state of constant transformation, with sustainability taking center stage in recent years. Numerous innovative sectors have emerged, including renewable energy, green finance, and the circular economy. Each technological and environmental advancement promises to reshape industries, yet the fundamental structure of…
Garvit Sahdev enjoys understanding ideas that shape our world. The Thoughtful Tangle is an initiative to share this journey and experience with his friends who love to do the same. He selects one idea and dives deep into it to understand its basics, relevance, impact and opportunities around it. The thoughtful tangle is special because 👇
📝 One long article per idea. We call it ‘The Basics’.
📝 Multiple unique insights. Separate small articles for special ‘Insights’.
🧑🤝🧑 Experts perspective. Check out ‘Insiders’
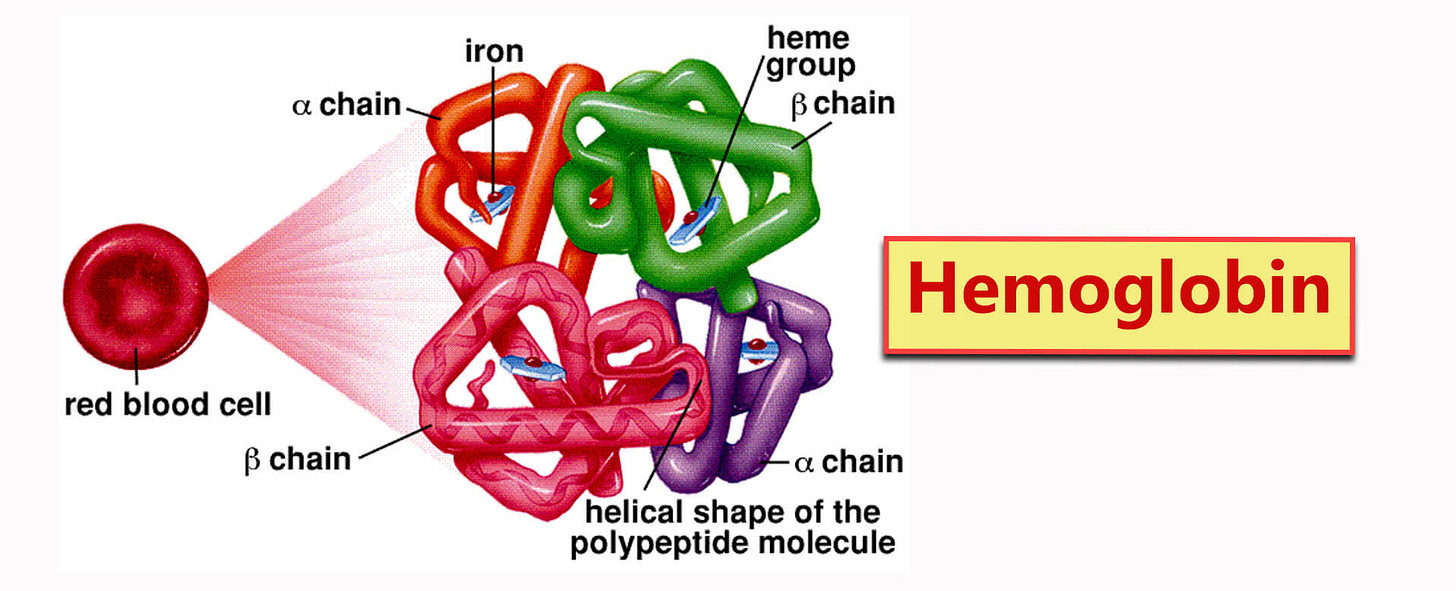
Haemoglobin
Haemoglobin is a protein in red blood cells responsible for transporting oxygen from the lungs to the body's tissues and returning carbon dioxide from the tissues back to the lungs.
It contains four heme groups, each capable of binding to one oxygen molecule.
Mechanism of Carbon Dioxide Transport
When blood travels through muscles and the brain, it becomes slightly alkaline due to certain chemicals on the surface of hemoglobin. This alkaline environment helps the blood absorb a lot of carbon dioxide from these tissues.
Later, when this blood reaches the lungs, something interesting happens. Hemoglobin grabs onto oxygen in the lungs and changes its shape. This change hides the specific chemicals (amines and imidazoles) inside hemoglobin. As a result, the blood's pH drops, which causes it to release the carbon dioxide it picked up earlier. This carbon dioxide then leaves your body when you breathe out. So, the blood's chemistry shifts to help your body get rid of carbon dioxide where it's not needed and absorb oxygen where it is needed.
Inspiration for Carbon Capture
Here's a simplified explanation of Hoshino's (Kyushu University) carbon capture system using hydrogel nanoparticles:
Hydrogels: These are materials made of crosslinked polymers that can hold a large amount of water (mimicking blood). Think of them as sponges that can absorb and retain water.
Amine Incorporation: Hoshino's team has added amines (compounds that can capture carbon dioxide) into the structure of these hydrogels. When these amines are in the hydrogel, they have a high pH, making them effective at capturing carbon dioxide (CO₂) from the environment. Similar amines are present in haemoglobin in red blood cells.
Temperature Response: The key innovation here is that the hydrogel nanoparticles respond to changes in temperature. (Haemoglobin structure in blood responds to oxygen)
At Cooler Temperatures: The hydrogel is water-attracting (hydrophilic). This means it absorbs water, swells up, and the amines are exposed and ready to capture CO₂ from the air. In blood the same activity happens when haemoglobin releases oxygen near tissues.
At Warmer Temperatures: The hydrogel becomes water-repelling (hydrophobic). This causes the hydrogel to collapse, squeezing out the water and hiding the amines inside the collapsed structure. This process releases the captured CO₂. In blood the same activity happens when haemoglobin absorbs oxygen in lungs.
Reusability: As the temperature cycles back down to cooler levels, the hydrogel returns to its original, swollen state, exposing the amines once again. This makes the system reusable for multiple cycles of carbon capture and release.
Comparison to Conventional Systems
In conventional systems used for capturing carbon dioxide (CO2) from smokestacks, such as aqueous amine solutions, the process involves two main steps: absorption and desorption.
Absorption: Aqueous amine solutions are exposed to flue gas (smokestack emissions) containing CO2. The amine molecules react with CO2, forming a chemical complex that removes CO2 from the gas stream.
Desorption: After the amine solution absorbs CO2, it needs to release the CO2 for storage or reuse. This is done through a process called desorption, which typically requires heating the amine solution to temperatures over 100 °C. At this temperature, the CO2 is released from the amine complex, allowing it to be captured separately.
The issue with this conventional method is that the desorption step is energy-intensive. Heating the amine solution to over 100 °C consumes a significant amount of energy, approximately 30% of the output of a typical power plant.
Now, regarding hydrogel-based systems:
Low Energy Requirements: Unlike conventional amine solutions that require heating to over 100 °C for CO2 release, hydrogels can achieve CO2 release at much lower temperatures (50–60 °C). This lower temperature requirement means that the energy needed for the desorption step is significantly reduced.
Utilization of Waste Heat: Many industrial processes generate waste heat at temperatures around 50–60 °C. Since hydrogel-based CO2 capture systems can operate effectively at these temperatures, the waste heat generated by industrial processes can be directly used to drive the desorption of CO2 from the hydrogel. This utilization of low-grade waste heat reduces the overall energy consumption and makes the process more energy-efficient compared to conventional methods.
Business Perspective
The idea of capturing carbon dioxide (CO₂) using hydrogel technology and converting it into useful products, like methane or bioplastics, makes business sense for several reasons:
Value Creation from Waste
Methane Production: CO₂ captured from power-plant emissions is converted into methane, a valuable gaseous fuel. This methane can be integrated into existing gas supply networks, like the domestic-gas-supply network. This creates a closed-loop system where waste CO₂ is transformed into a usable energy source, reducing dependence on fossil fuels.
Bioplastic Synthesis: Collaborating with synthetic biologists, the captured CO₂ can be used to produce high-value bioplastics and aviation fuel. These products have growing markets as industries seek sustainable alternatives to traditional plastics and fossil fuels.
Cost Advantage
Carbon Emissions Reduction: By capturing and converting CO₂, the process helps reduce greenhouse gas emissions from power plants and similar sources at reduced cost. This can help companies meet regulatory requirements and avoid potential fines or penalties associated with excessive emissions.
Space Exploration Applications
Spacecraft CO₂ Capture: Partnering with JAXA to develop a CO₂ capture system for spacecraft highlights the system's adaptability to extreme environments. Efficiently capturing exhaled CO₂ in humid conditions without the need for air drying can significantly reduce energy consumption, which is crucial in space missions.
My Perspective
This technology has made carbon dioxide capturing, transportation, and concentrated release easier and cheaper. Capturing can be done at most concentration levels. Transportation is easy as it is a gel. The low energy-intensive concentrated release can reduce the price of Carbon dioxide in the markets.
If it is executed successfully at scale, it will become major part of carbon dioxide supply chain.
References:
Capturing carbon by mimicking haemoglobin (nature.com)
SOMEONE CAN BENEFIT FROM IT
If you find this post helpful, we would be grateful if you could take a moment to share it with others who might benefit from it. Your kindness and support mean a lot to us.
DYNAMIC CONTENT
No one knows how deep a rabbit hole can go.
All our posts are regularly updated as soon as new information becomes available. We also keep note of the questions asked by our readers and add their answers to the article. So, stay curious and ask questions in the comments.
ABOUT THE AUTHOR
Hey everyone, I'm Garvit Sahdev 😎. I'm on a mission to gain a deeper understanding of the world, and to develop solutions that can trigger significant global change.
My curiosities span various domains including food, business theories, material science, market size calculations, economics, politics, and sports, etc. 🧐
Professionally, I have a diverse background spanning startups, consulting, policy development, market research, system building, ISO, colour physics, nanomaterial synthesis, textile chemistry, etc. 🐘
Thanks for reading The Thoughtful Tangle! Subscribe to continue reading deeply researched stories about the interesting concepts that shape our world. ❤️